December 1, 2012 (Vol. 32, No. 21)
Rashi Takkar
HPLC has long been regarded as the standard for antibody quantitation. It is extensively used in drug development processes to quantitate antibodies in cell culture supernatants, fed-batch flasks, and bioreactors. However, HPLC quantitation cannot be performed in crude matrices, is unable to detect low titers, suffers from low throughput, and requires extensive cleaning and maintenance. In contrast, the Octet platform offers a microfluidics-free, higher throughput solution to meet quantitation needs with accuracy, precision, specificity in crude matrices, and a wider dynamic range for detection of low to high titers.
ForteBio’s Octet platform (Figure 1) provides comprehensive characterization across a range of applications in various stages of drug development, including antibody and protein quantitation, kinetic characterization of proteins and small molecules, and validation of reagents. The Octet platform circumvents the limitations of ELISA and HPLC analysis, enabling informed decisions to be made earlier in bioprocess development.
Octet systems perform sample analysis in 96- and 384-well microplate formats, processing up to 16 samples in parallel. The simple Dip and Read™ approach enables streamlined workflows and rapid quantitation of 96 samples in as little as 20 minutes, or 384 samples in 70 minutes.
Octet systems measure binding signal using a proprietary optical technology called Bio-layer interferometry (BLI). Biosensors coated with capture molecules dip in to samples and the concentration of the target antibody in the sample is measured via a direct binding assay. In a typical quantitation assay, a standard curve is generated using known amounts of the antibody, and unknown sample concentrations are extrapolated from the standard curve.

Figure 1. Bio-layer Interferometry (BLI)-based Octet and BLItz Instruments
Comparison of HPLC
Octet quantitation data compares well to values determined by techniques such as HPLC, ELISA, and A280 spectrophotometry. The values have been shown to be accurate and precise over a broad dynamic range of concentrations, even in crude samples (Figure 2).
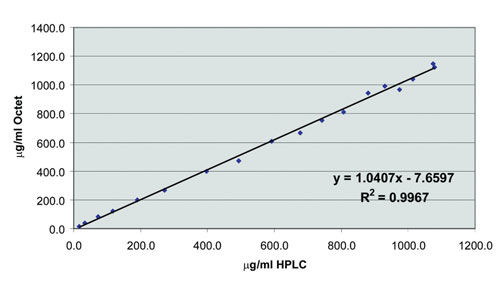
Figure 2. Excellent correlation of Octet data with HPLC values over a broad dynamic range.
Instrument Selection
To meet different analytical requirements, several systems based on BLI technology are available. The Octet RED96 and RED384 instruments provide a large dynamic range of detection of human IgG from 25 ng/mL to 2 mg/mL using Protein A biosensors. Octet QKe and QK384 systems detect antibody concentrations from 100 ng/mL to 700 µg/mL. Octet RED384 and QK384 instruments can be easily integrated with robotic systems for automated liquid handling, enabling the screening of thousands of samples in a few hours.
The BLItz™ system is ideal for quick checks of protein presence in crude matrices as well as instant determination of protein breakthrough at various stages of purification. BLItz is a highly compact and affordable single-channel instrument that analyzes proteins in a 4 μL drop of sample.
Biosensor Selection
A variety of biosensors are available from ForteBio for quantitation of antibodies and other proteins with various capture molecules including: anti-human IgG Fc, anti-murine IgG Fv, protein A, protein G, protein L, anti-penta-His, streptavidin, anti-human Fab-CH1, anti-GST, and Ni-NTA.
These biosensors exhibit high specificity and are designed to perform titer measurements accurately in the presence of host cell proteins and debris, greatly simplifying analysis at all stages of development.
Assay Development
Development of an Octet assay for in-process testing is both rapid and simple. Relative to techniques like ELISA or HPLC, Octet assays require fewer reagents, consumables, assay steps, and does not require labeling. In particular, Octet users highly value the reduced number of sample preparation steps, such as not having to purify or dilute samples. This not only saves hands-on time but also greatly improves the accuracy and precision of assay results.
Since BLI signal detection occurs in response to interactions at the tip of the biosensor, changes to the matrix and unbound proteins in solution, such as those found in crude lysates, have minimal effect on the signal. In rare cases where nonspecific binding of irrelevant proteins is observed on the biosensor, a simple reference subtraction can be applied in the Octet software to correct for such effects. In addition, with Octet data analysis, previously saved standard curves can be imported into new data sets to quantitate unknown samples.
User cost-analysis has determined that with the labor and time savings Octet assays provide, the price per sample (including equipment costs) was equivalent or lower than ELISA and HPLC. In addition, with Octet assays many biosensors can be regenerated and used multiple times, enabling even greater cost per sample savings. Biosensor regeneration is completely automated by the Octet platform and does not require any additional user involvement.
GLP Compliance
The Octet platform includes IQ/OQ tools and FDA 21 CFR Part 11 compliant software tools to meet GLP requirements. Users may choose IQ/OQ service be performed by ForteBio qualified service personnel, or by third-party service personnel using the ForteBio IQ/OQ service kit. 21 CFR Part 11 compliant software tools provide electronic security, audit trails, user access management, and other compliance features.
Conclusion
The Octet platform provides an automated solution for maximizing the efficiency of antibody quantitation in biopharmaceutical development processes. A variety of assay types and conditions can be developed to suit various analytical requirements. The Octet platform’s simple Dip and Read approach enables high throughput, accurate, and highly precise quantitation of monoclonal antibodies at concentrations relevant to the entire drug development, QC, and manufacturing processes while requiring less hands-on time.
ForteBio, A Division of Pall Life Sciences
Rashi Takkar
Applications Scientist







