September 15, 2013 (Vol. 33, No. 16)
New Methodology Shown to Improve Screening
There is increasing evidence that 3D cell culture models are more physiologically relevant than traditional 2D cell cultures, particularly in the context of in vitro tumor modeling. Spheroid cultures, which are self-assembled microscale 3D aggregates of cells, have vast applications in drug discovery in that they are easy to form and contain few extraneous variables, such as artificial matrices. Furthermore, spheroids possess physiological cell-to-cell contacts, secrete their own extracellular matrix (ECM), and have nutrient, drug, and oxygen mass transfer gradients similar to those that exist in vivo.
The use of spheroids in preclinical drug discovery has potential for generating more biologically relevant data before the animal and human trial phases. Previously, two major roadblocks in the use of 3D spheroid cultures for drug testing were a lack of (1) a spheroid model in a reproducible and scalable high-throughput screening (HTS) format, and (2) a robust and reliable method for analysis of such 3D spheroid cultures.
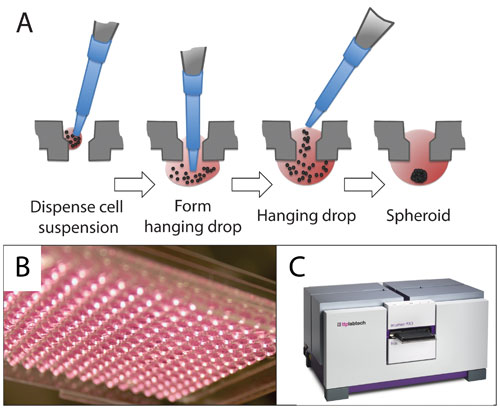
3D spheroid cultures generated in the Perfecta3D® Hanging Drop Plates (HDPs) from 3D Biomatrix were used to create a colorectal cancer model for a proof-of-concept drug-validation experiment. The Perfecta3D HDPs are designed for easy, uniform, and controllable spheroid culture in a 96- or 384-well plate format. A drop of cell suspension is pipetted into the top of each well. The plate geometry allows the drop to hang stably below the well where the cells aggregate into a spheroid over one to several days (Figures 1A and 1B). One spheroid forms per well; the spheroid size is controlled by the number of cells seeded.
Figure 1. Perfecta3D HDP and acumen eX3: (A) 96- and 384-well Perfecta3D Hanging Drop Plates facilitate the culture of spheroids within a hanging drop. The user pipettes the cell suspension into each well, and the spheroid self-assembles. (B) Drops hang stably below each well. (C) The acumen eX3 was used to image and quantify cellular viability in spheroids before and after drug treatment.
The workflow illustrated in Figure 2 demonstrates how 3D spheroid cultures were paired with high-content fluorescence imaging to generate and analyze an in vitro model for drug testing. Spheroids were imaged for viability pre- and post-drug treatment with the acumen® eX3 from TTP LabTech, a high-content imager (Figure 1C). The acumen high-content imager performs multicolor, whole-well imaging and is unique in that its depth of field is sufficient to capture spheroids without the requirement of assembled Z-stacks. In the platform described here, the acumen high-content imager allowed for the robust and rapid quantification of spheroid formation.

Figure 2. Drug testing workflow utilizing high-content analysis: Spheroids can be formed in Perfecta3D Hanging Drop Plates and analyzed via high-content and traditional methods before and after treatment with selected drug compounds.
3D Spheroid Model Formation and Analysis
HCT116 human colorectal carcinoma cells were used to generate spheroids by seeding 20,000 cells in 96-well HDPs and 5,000 cells in 384-well HDPs. Cells were maintained in hanging drops for three days to permit spheroid formation. The resulting spheroids were uniformly sized and displayed characteristic morphologies as observed by light microscopy (Figure 3A).
Spheroids were transferred into 384-well, black-sided, clear-bottomed, high-content imaging assay plates (Greiner) by the addition of supplementary media. Viable cells were stained with calcein AM according to the manufacturer’s instructions. Spheroid size analysis (Figure 3B) was performed by capturing images via confocal microscopy.
The resulting spheroids had an average diameter of 650 µm from 96-well HDPs and 350 µm from 384-well HDPs (Figure 3B), demonstrating excellent reproducibility of spheroid size relative to initial cell plating number.
To gauge the applicability of 3D spheroids as a tumor model, sectioning and immunohistochemistry were performed to further characterize the tumor-like morphologies (hematoxylin and eosin stain, H&E), proliferative zones (Ki67 antibody), and presence of hypoxic adducts (pimonidazole, PIMO) of 384-well tumor spheroids. Although the spheroids were less than 500 µm in diameter, there was evidence of hypoxic cores, and proliferation was detected throughout the spheroids, particularly along the outermost layer (Figure 3C).
The ability to quickly and easily create multiple 384-well plates of spheroids with consistent size and biologically relevant microtumor physiology allows for high-throughput screening in 3D. Based on these observations, HDP-derived spheroids constitute a robust 3D tumor model for high-throughput drug discovery, especially when employing the acumen high-content imager for quantifying spheroid formation.
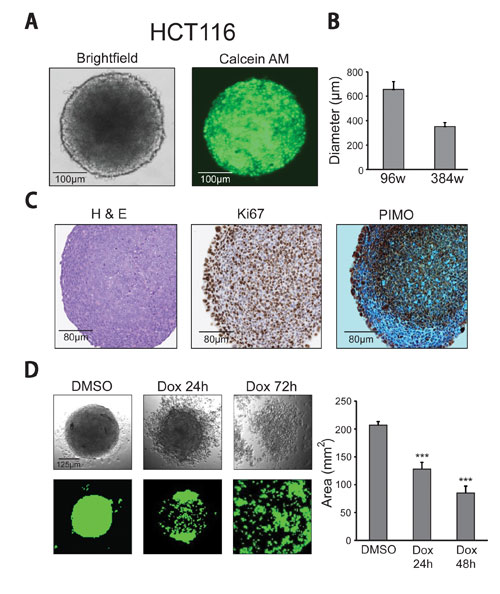
Figure 3. 96- and 384-well spheroid formation, imaging, drug treatment, and analysis: (A) HCT116 human colorectal carcinoma spheroids from 384-well HDPs display characteristic morphology under bright-field microscopy and can be stained with calcein AM for fluorescence-based size and viability analysis. (B) 384-well spheroids were on average 350 µm, and 96-well spheroids measured approximately 650 µm. Errors bars represent standard deviations. (C) Immunohistochemistry sections of 384-well HCT116 colorectal carcinoma spheroids were stained with H&E, Ki67 (proliferation), and PIMO (hypoxic adducts). (D) Bright-field microscopy and acumen images of calcein AM-stained HCT116 colorectal carcinoma spheroids treated with 1.1 µM Dox or DMSO at 24- and 72-hour time points. Bar graph shows data representative of Dox effects on spheroid cell viability at 24 and 48 hours as measured by total calcein AM positive area recorded on the acumen eX3. Errors bars represent standard deviations (*** = p = .01).
3D Spheroid Model Drug Treatment, Imaging, and High-Content Analysis
To illustrate the utility of the HDP/acumen system for drug screening, HCT116 colorectal carcinoma spheroids were treated with a commonly used anticancer agent Doxorubicin (Dox) in dimethyl sulfoxide (DMSO) or with DMSO alone as vehicle control. Treatment consisted of 1.1 µM Dox or DMSO for up to 72 hours. At 24-, 48-, and 72-hour time-points, calcein AM was added, as described previously.
Spheroid cell viability was quantified on the acumen high-content imager by capturing the total area of calcein AM signal per well. Spheroid cell viability was significantly reduced at both 24 and 48 hours of Dox treatment compared to the vehicle control (Figure 3D). HDP-derived spheroids and the acumen high-content imager can be used harmoniously to accurately and easily assess drug cytotoxicity in 3D spheroid models of disease by HTS methods.
Conclusions
HCT116 human colorectal carcinoma spheroids produced with both 96- and 384-well Perfecta3D HDPs were uniformly sized. Spheroids formed in 384-well HDPs possess biologically relevant morphologies in that normal matrix distribution was observed, proliferation zones were minimal, and a significant hypoxic core was seen. The spheroids were subjected to a proof-of-concept HTS scenario, and demonstrated robust and reproducible spheroid phenotypes when assayed on the acumen high-content imaging system.
Drug developers are searching for more physiologically relevant models to use in preclinical drug discovery and screening to reduce costs, achieve more robust data, and minimize discrepancies observed between 2D screens and small animal trials. The 3D spheroid represents one of the most promising models for screening because of the ease of culture, simplicity of outcome measures, consistency of results, and overall similarity of physiology to tumors in vivo.
Combining the Perfecta3D HDPs and the acumen eX3 high-content imager, creating a tool for formation and analysis of a 3D spheroid model of colorectal cancer, has great potential for drug discovery screening. Use of HDPs and the acumen high-content imager improve the preclinical drug screening process and allow for more efficient triaging of the best preclinical candidates.
Shane R. Horman, Ph.D., is a principal investigator, Jeremy To is a research associate, and Anthony P. Orth, Ph.D., is associate director of genomics at the Novartis Research Foundation. Nicky Slawny, Ph.D., is applications director and Meghan Cuddihy, Ph.D. ([email protected]), is marketing director at 3D Biomatrix. Diana Caracino, Ph.D., is U.S. Field Applications Specialist at TTP Labtech.