January 1, 1970 (Vol. , No. )
Sanjin Zvonic, Ph.D.
Developing with commercialization in mind is key.
Developing a cell therapy presents a unique set of challenges, even for the world of biotechnology. As a developer, the most important guidance is to develop with commercialization in mind. If your product cannot be commercialized successfully, neither your company nor your target patient population will benefit. Here are ten tips on what “developing with commercialization in mind” means in practice:
- Product characterization: Although only basic characterization is required in initial phases of development, mature/commercial products will require a specific safety/identity/purity/potency characterization profile. Plus, characterization is crucial for bridging ongoing development efforts/changes, to existing processes. Thus, it is crucial to start collecting data necessary to develop these specifications from the very beginning of development, otherwise, it may be very challenging to develop a commercially acceptable profile “from scratch” at Phase II and later.
- Cellular source material: From the very beginning of development, you should place a strong focus on characterizing and standardizing cellular source material, its collection, donor profile, and acceptance criteria. Otherwise, the variability of cell material will result in greater product lot-to-lot variability and lot failure rates.
- Product stability: Stability is crucial for commercial success of a product, as it will dictate the product distribution and delivery logistics/requirements. Greater stability provides greater delivery flexibility, so consider how the product can be designed with stability in mind.
- Distribution: Driven by stability and final formulation, distribution logistics need to be considered from early stages. These logistics will dictate the manufacturing model (centralized vs. multi-center), as well as clinical-site model (direct delivery vs. clinical-site manipulation). From a risk management and resource perspective, it is advisable to minimize or eliminate clinical-site manipulations and reduce the number of manufacturing sites (See Box). Keep in mind that, for a cell therapy, “delivery” means much more than “shipping”.
- Automation: Automation is essential for driving down product manufacturing costs and achieving price points suitable for today’s marketplace. Thus, from the beginning stages, you should strive to identify points in your manufacturing process that would be suitable for automation later on, and include these considerations in product development. This way, automation at a later Phase is more seamless and less risky.
- Scale-up/Scale-out: As with automation, you need to consider future scale-up/scale-out needs and build them into early-phase development. This will minimize the risk of having to “backtrack” upon implementation of large-scale solutions.
- Raw material comparability: Raw materials should have specifications and testing/qualification programs that allow the developer/manufacturer to use interchangeably several variants of each material, while maintaining the product characterization profile. This practice minimizes the risks associated with any future changes in material supply or geographic market variations in material availability and regulatory compliance.
- Robustness of the supply chain: Robustness should be evaluated based on material manufacturer track record, business stability and future direction, regulatory compliance, flexibility/robustness of quality systems, manufacturing output and lead times, and coverage of multiple geographical markets. For critical materials, manufacturers should agree to provide the rights to manufacture the material/reagent should they themselves choose to discontinue it.
- Cost of goods: Streamline your manufacturing and analytical resource requirements through development, scale-up/out, and automation in order to achieve a notable reduction in the cost of goods. Indeed, the very business model chosen has significant impact on COGs.
- Cost of manufacturing and delivery: Streamline the manufacturing and delivery processes via limited manufacturing sites and minimal clinical-site manipulation requirements to decrease costs. Furthermore, consider outsourcing the manufacturing and delivery functions, in lieu of investing in in-house infrastructure and its maintenance, to achieve significant cost and risk mitigation.
Box: Resource Demand During Commercialization
This chart offers perspective on how the choice of delivery and manufacturing model impacts resource requirements. An advantage is achieved by utilizing a multi-step model over direct delivery due to decreased clinical site resource requirements. A further advantage is achieved by choosing a multi-step delivery/centralized manufacturing hybrid model by further reducing the clinical site resources required.
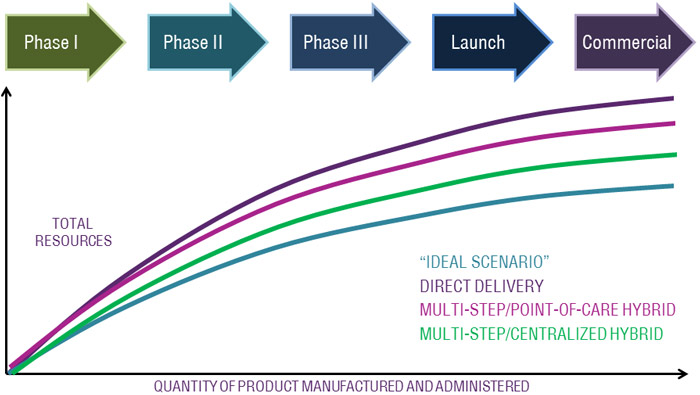
Chart: Resource demand during commercialization
Sanjin Zvonic, Ph.D., is director, technology and business development, at PCT (Progenitor Cell Therapy). For more information on how to develop, manufacture, deliver, and commercialize your cell therapy product, please visit www.pctcelltherapy.com.